FDA Approves Licence For Alzheimer’s Drug Aducanumab In US
The US Food and Drug Administration (FDA) has announced that it has granted a licence for the drug aducanumab (known commercially as Aduhelm) in people with Alzheimer’s disease in the US, with expectation of a further stage four clinical trial. This marks the first time a drug has been approved for dementia treatment in nearly 20 years.
The ruling will see aducanumab, an antibody treatment developed by the pharmaceutical company Biogen, made available to patients in the US. It was approved via the Accelerated Approval pathway. Accelerated approval can be based on the drug’s effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients, with a required post-approval trial to verify that the drug provides the expected clinical benefit. If the drug does not work as intended, they can take steps to remove it from the market.
The UK regulator, the MHRA, and EU regulator, the EMA, are expected to give their decisions on whether to grant the drug a similar or different form of licence this autumn.
Alzheimer’s Research UK has today written to the Health Secretary Matt Hancock to request that the evaluation process by UK authorities be accelerated.
There are an estimated 850,000 people in the UK living with dementia, most commonly caused by Alzheimer’s disease, and it is the leading cause of death in England. With an ageing population and currently no treatments available to delay the onset or reduce the progression of diseases that cause dementia, this number is set to rise to 1.3 million by 2030.
How does aducanumab work?
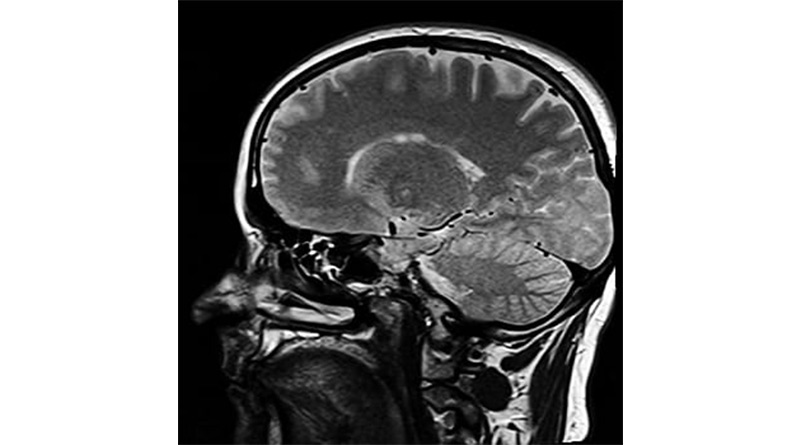
Aducanumab is designed to target amyloid, one of the hallmark proteins that builds up in the brains of people with Alzheimer’s at an early stage in the disease process. The drug is given through an infusion into a person’s arm and was tested in people who have mild cognitive impairment (MCI) and very early stage of Alzheimer’s who have had amyloid detected in their brains using a PET scan. However, early information suggests the FDA’s licence appears to be broad enough to cover anyone with Alzheimer’s disease, with the FDA highlighting uncertainties around the clinical benefit.
In 2019, Biogen stopped its two phase III trials of aducanumab (called EMERGE and ENGAGE) because early indications suggested the drug would not show sufficient benefit to patients. However, after further analysis the drug company announced they were filing for FDA approval based on results from one of the studies showing a noticeable slowing of cognitive decline in people on higher doses of the drug.
Over recent months, the trial results and submission have sparked debate about how regulators judge the effectiveness of any new Alzheimer’s treatment, and how meaningful the impact of a drug would need to be on someone’s day-to-day life to meet that threshold.
In November 2020 the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee voted against approval of aducanumab over concerns that the evidence did not support its effectiveness. However, in today’s ruling the FDA have stated that they will assess the further evidence of benefit in the post-approval clinical trial and as greater numbers of people receive the treatment.
Hilary Evans, Chief Executive at Alzheimer’s Research UK, said:
“Today’s decision by the FDA marks a pivotal moment in the search for life-changing new treatments for Alzheimer’s disease. The approval is a positive step forward for people with Alzheimer’s disease in the US and we welcome the opportunity for Biogen to conduct a post-approval clinical trial to reveal more about the potential real-world benefits of aducanumab. Aducanumab will be the first ever drug to reach patients in the US that tackles the underlying disease process itself. The findings of these additional studies could pave the way for a new generation of life-changing drug treatments.
“People with dementia and their families have been waiting far too long for life-changing new treatments. It is now essential that regulatory authorities here assess the evidence to decide whether they believe the drug is safe and effective for use in the UK. Alzheimer’s Research UK has today written to the Health Secretary Matt Hancock calling on the government to prioritise and accelerate this process, to give people with Alzheimer’s the answers they need as quickly as possible.
“We hope this historic ruling has an immediate positive impact on the global search for effective dementia treatments. Aducanumab was only tested in certain individuals with early Alzheimer’s, so renewed focus and investment in dementia research will speed up the search for life-changing treatments for those with other dementias, and in the later stages of disease.”
“Aducanumab is still some way from reaching patients in the UK, but Alzheimer’s Research UK continues to work tirelessly to prepare the UK health system so that any new drug can reach those who need it most without delay. Research has the potential to transform the lives of everyone affected by dementia and we will keep working to make more breakthroughs