EMA Committee Refuses Licence for Alzheimer’s Drug Aducanumab in EU
A key committee for the European Medicines Agency has recommended refusing an application by the pharmaceutical company Biogen for a licence for the Alzheimer’s drug aducanumab.
The Committee for Medicinal Products for Human Use (CHMP), which reviewed Biogen’s application to license the drug in the EU, made its recommendation on the basis that results from the main clinical studies were conflicting and did not show overall that aducanumab was effective at treating adults with early-stage Alzheimer’s disease. The committee’s official recommendation will now be passed to the European Commission to be ratified.
This follows a decision by the US Food and Drugs Administration (FDA) in June 2021 to approve the drug for use in the US in people with mild cognitive impairment (MCI) or mild Alzheimer’s disease. It is not yet known whether Biogen will submit a separate application to the UK regulator, the Medicines and Healthcare Products Regulatory Agency (MHRA), to seek a licence for aducanumab in the UK.
There are estimated to be nearly a million people in the UK living with dementia, with most of those living with Alzheimer’s disease, and the condition is one of the country’s leading causes of death. With an ageing population and limited treatment options for the diseases that cause dementia, the number of people with dementia is currently expected to rise to 1.3 million by 2030.
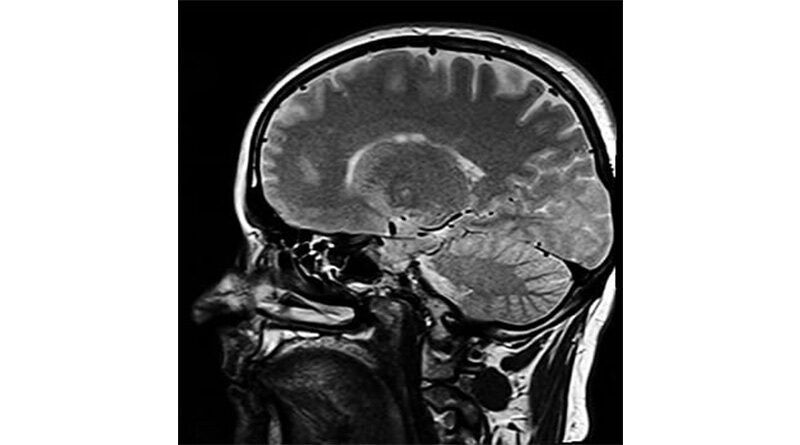
Aducanumab, known commercially as Aduhelm, is designed to target amyloid, one of the hallmark proteins that builds up in the brains of people with Alzheimer’s at an early stage in the disease process.
In today’s ruling the CHMP have stated that although aducanumab reduces amyloid in the brain, the link between this effect and clinical improvement had not been established. In addition, their view was that the studies did not show aducanumab was sufficiently safe, as images from brain scans of some patients showed abnormalities suggestive of swelling or bleeding, which could potentially cause harm.
Hilary Evans, Chief Executive at Alzheimer’s Research UK, said: “Today’s announcement will come as bitterly disappointing news for people with Alzheimer’s disease.
“The last year has seen a complex situation with aducanumab play out differently through EU and US regulatory pathways. What people with Alzheimer’s need is a medicine that improves their lives and the EMA committee’s decision reflects a lack of clear evidence that aducanumab can do that.
“We understand why the committee couldn’t recommend a licence for aducanumab at this time, but we must also recognise the many thousands of people with Alzheimer’s who continue to be without effective new treatment options.
“The manufacturer, Biogen, must continue collecting essential data to clarify the safety and effectiveness of aducanumab. While further data collection is happening in the US, the UK is uniquely well placed to deliver world-class clinical studies that could address unanswered questions about the drug.
“It’s a combination of many research advances that brings any drug to this pivotal point and today’s news must be a catalyst for renewed urgency and investment. With over 125 Alzheimer’s drugs currently in clinical trials, the government, regulators and life science companies must urgently work together to speed up UK access to future medicines. Alzheimer’s Research UK continues to help prepare the UK health system so that future life-changing treatments can reach patients without delay.”